Quantum Numbers Khan Academy
Quantum Numbers Khan Academy - If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. Explains that only two electrons. Learn how quantum numbers are. Learn how quantum numbers are. In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons.
Learn how quantum numbers are. Learn how quantum numbers are. In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. If you're seeing this message, it means we're having trouble loading external resources on our website. Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. Explains that only two electrons. If you're behind a web filter, please.
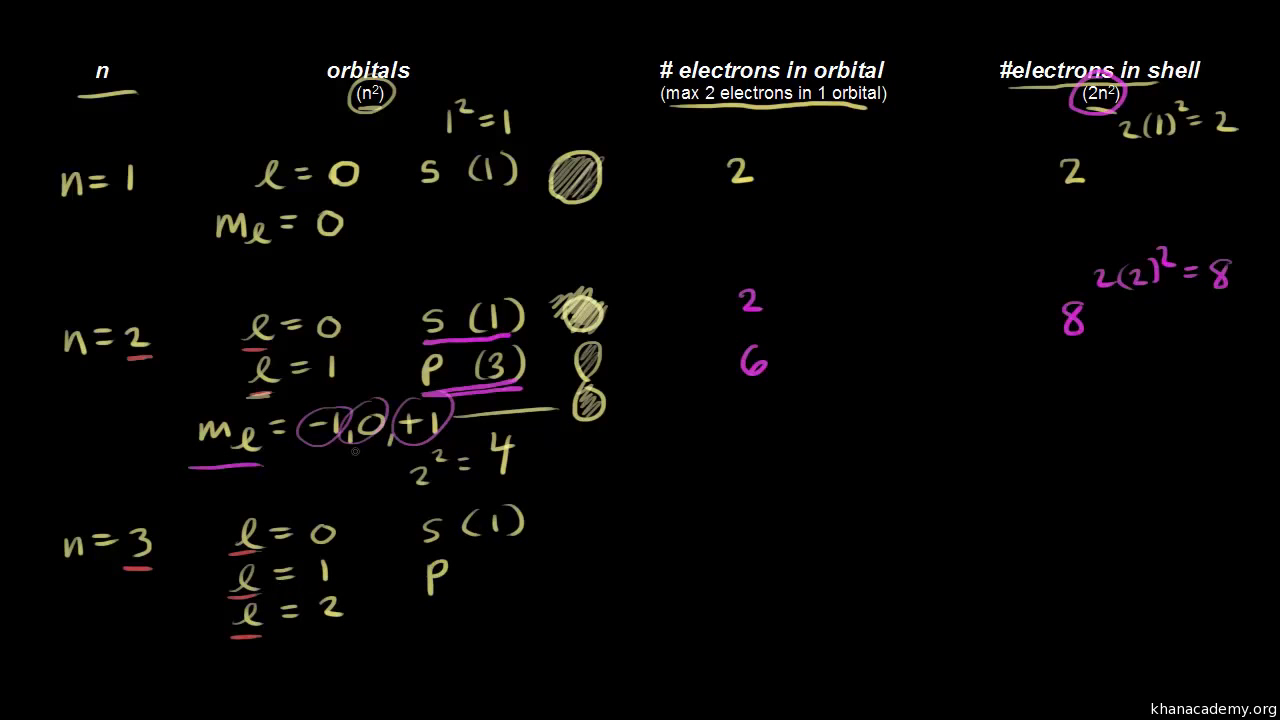
Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please. Explains that only two electrons. Learn how quantum numbers are. Learn how quantum numbers are. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system.
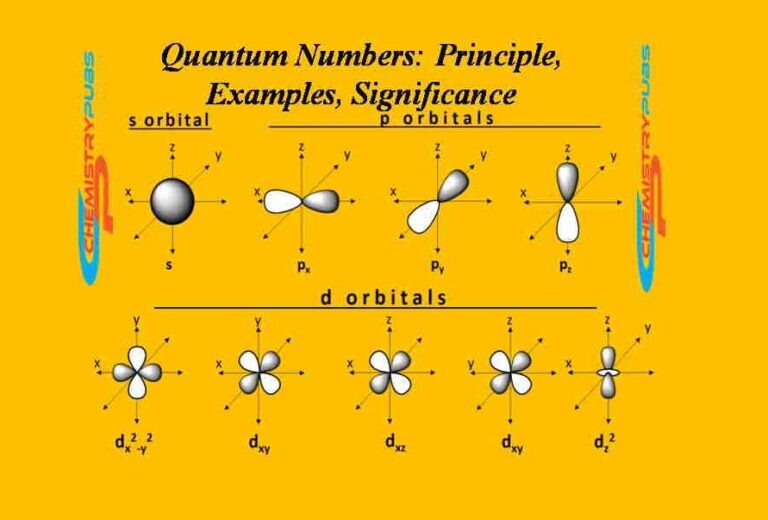
Quantum Numbers Principle, Examples, Significance Chemistrupubs
In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Learn how quantum numbers are. If you're behind a web filter, please. Definition of orbital as region of high probability for finding electron, and how.
Quirky Quantum Fields Khan Academy Breakthrough Junior Challenge 2020
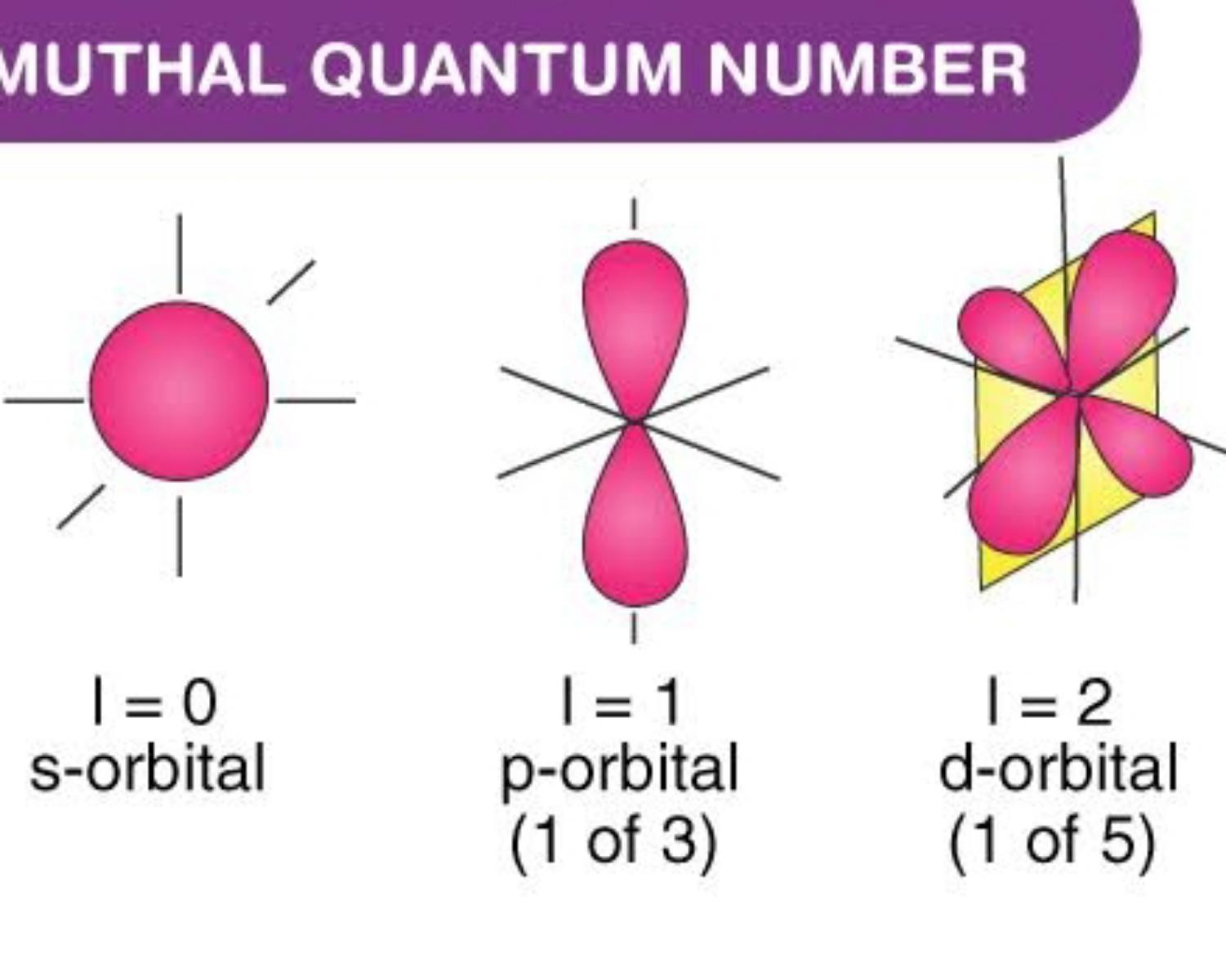
Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Different types of orbitals.
Quantum Numbers Diagram
Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Learn how quantum numbers are. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Explains that.
Quantum numbers Match up
Explains that only two electrons. Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Definition.
How To Write Quantum Numbers For Elements
Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Explains that only two electrons. Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. If you're behind a web filter, please. Learn how quantum numbers are.
Quantum Numbers Section ppt download
Learn how quantum numbers are. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. If you're seeing this message, it means we're having trouble loading external resources on our website. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. If you're behind.
Spin Quantum Numbers (ms) Deepstash
Learn how quantum numbers are. Learn how quantum numbers are. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. If you're behind a web filter, please.
The quantum mechanical model of the atom Quantum numbers and orbitals
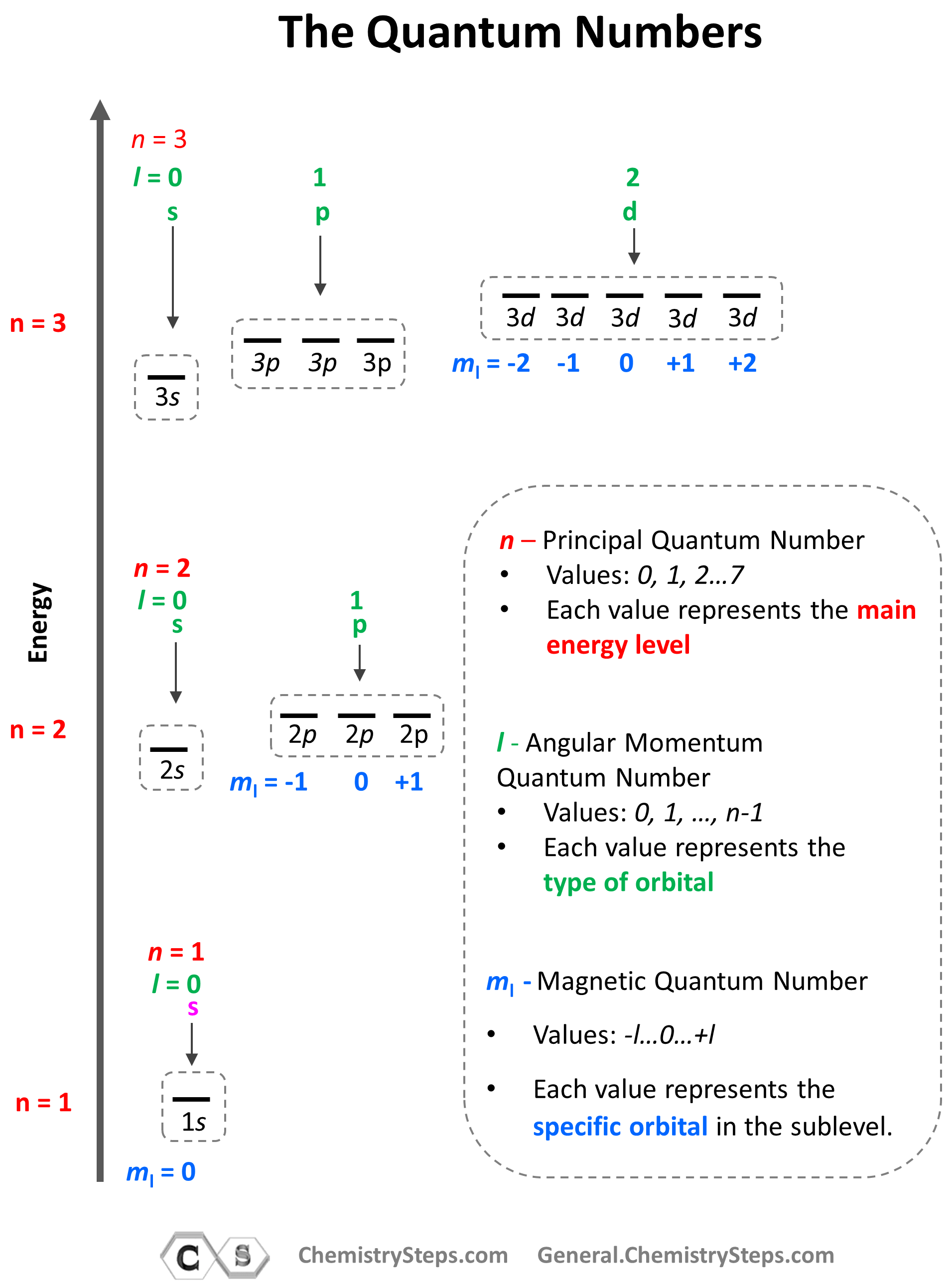
If you're behind a web filter, please. Learn how quantum numbers are. Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. In quantum physics and chemistry, quantum numbers are quantities.
Electron configurations for the first period Chemistry Khan Academy
If you're seeing this message, it means we're having trouble loading external resources on our website. Learn how quantum numbers are. Explains that only two electrons. Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. Different types of orbitals (s, p, d, f) have different shapes and can.
SOLUTION Quantum numbers Studypool
In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Learn how quantum numbers are. If you're seeing this message, it means we're having trouble loading external resources on our website. Calculates number of orbitals.
Learn How Quantum Numbers Are.
Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. If you're seeing this message, it means we're having trouble loading external resources on our website. Explains that only two electrons. If you're behind a web filter, please.
In Quantum Physics And Chemistry, Quantum Numbers Are Quantities That Characterize The Possible States Of The System.
Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Learn how quantum numbers are.